What Is Addition Reaction Give An Example
Published on July 30 2020 by Pritha Bhandari. I was wondering if you could perhaps give a generic mechanism or a similar example.

Nucleophilic Addition Definition Example And Mechanism
Mean median and mode.

What is addition reaction give an example. Measures of central tendency help you find the middle or the average of a data set. We would like to show you a description here but the site wont allow us. Many of the organic reactions involve.
Upon addition of acid the oxygen is protonated Step 2 arrows C and D to give. Revised on October 26 2020. These new chemical species can fall apart change to new structures combine with each other or other molecules or.
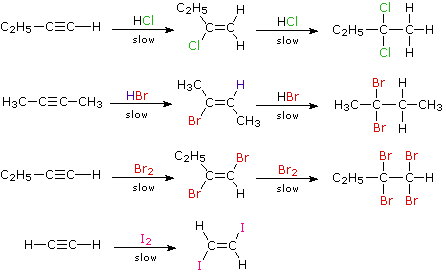
HCl CH 2 CH 2 CH 3 CH 2 Cl. The DielsAlder reaction with 13-dienes give 14-cyclohexadienes. This general reaction has been extensively developed.
In the reaction mechanism for the Schmidt reaction of ketones the carbonyl group is activated by protonation for nucleophilic addition by the azide forming azidohydrin 3 which loses water in an elimination reaction to diazoiminium 5. One of the alkyl or aryl groups migrates from carbon to nitrogen with loss of nitrogen to give a nitrilium intermediate 6 as in the Beckmann rearrangement. I note you give two examples of lactose and maltose as disaccharides that are reducing sugars as they contain the hemiacetal.
I have been searching for a reaction mechanism for the Amadori adduct formed from dexamphetamine and lactose. The cycloadduct derived from the addition of alkynes to 2-pyrone eliminates carbon dioxide to give. In an addition reaction the components A and B are added to the carbon-carbon multiple bonds and this is called addition reaction.
For another example of this see Ring-Chain Tautomerism. Or carbonate CO 3 2It also includes analogous behaviour of molecules and ions that are acidic but do not. Electrophilic alkynes are especially effective dienophiles.
Or acetic acid CH 3 CO 2 H or electrically charged ions such as ammonium NH 4. Acidbase reaction a type of chemical process typified by the exchange of one or more hydrogen ions H between species that may be neutral molecules such as water H 2 O. Photochemical reaction a chemical reaction initiated by the absorption of energy in the form of lightThe consequence of molecules absorbing light is the creation of transient excited states whose chemical and physical properties differ greatly from the original molecules.
The 3 most common measures of central tendency are the mode median and mean. In the reaction given below when HCl is added to ethylene it will give us ethylene chloride. NaBH 4 is a source of hydride H- and the reaction begins with the addition of hydride to the carbonyl to the aldehyde Step 1 arrows A and B.
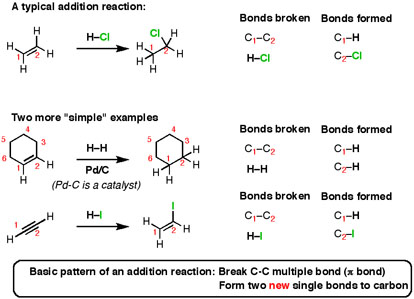
27 1 Organic Reactions An Introduction Chemistry Libretexts
Addition Reaction Of Alkenes With Halogens Easy Exam Revision Notes For Gsce Chemistry

Cis Product In An Anti Addition Reaction Of Alkenes Chemistry Steps

Addition Reaction Definition Examples And Mechanism

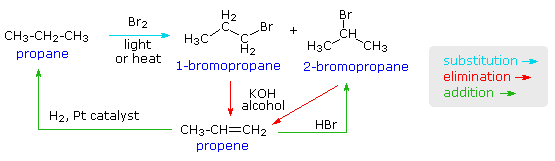
Reactions Of Alkenes And Alkynes Introduction To Chemistry

Addition Reaction Definition Examples And Mechanism

Addition Reaction Definition Examples And Mechanism

Addition Reaction Definition Examples And Mechanism

How Can A Cis Product Be Obtained From An Anti Addition Reaction Chemistry Reactions Organic Chemistry
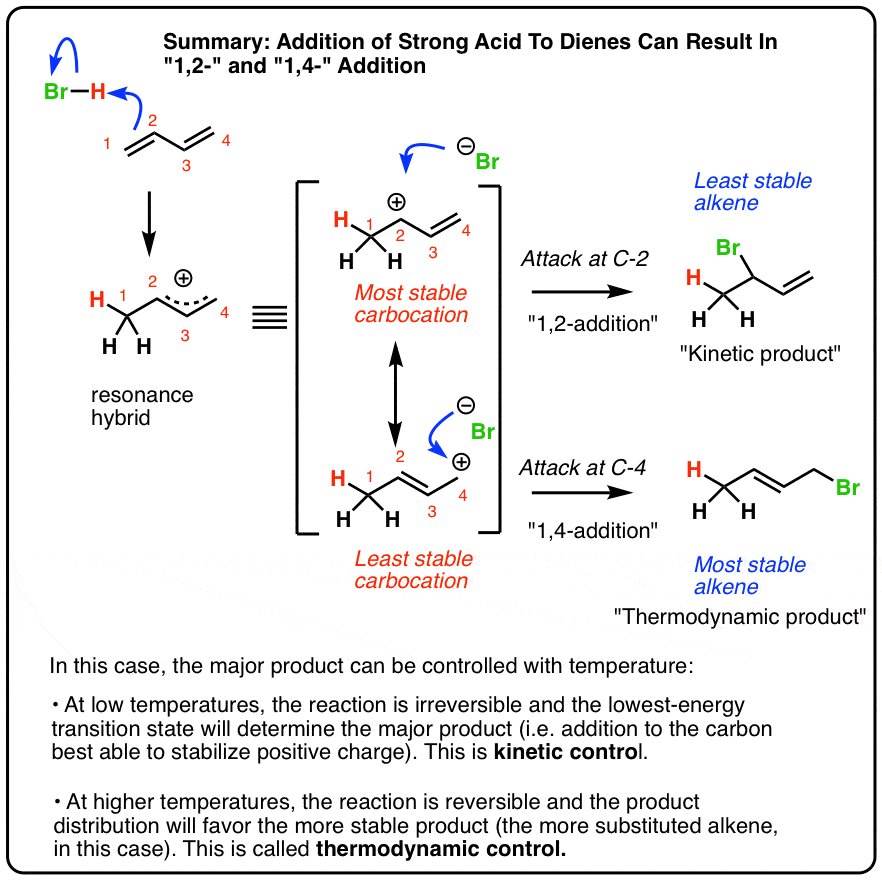
Reactions Of Dienes 1 2 And 1 4 Addition Master Organic Chemistry

Addition Reaction Definition Examples And Mechanism
Addition Reaction Of Alkenes With Halogens Easy Exam Revision Notes For Gsce Chemistry
Difference Between Addition And Substitution Reactions Definition Types Characteristics Examples Comparison
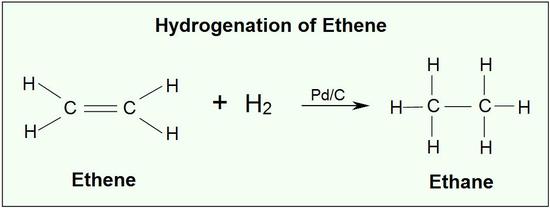
Catalytic Hydrogenation Of Alkenes Chemistry Libretexts
Difference Between Addition And Substitution Reactions Definition Types Characteristics Examples Comparison
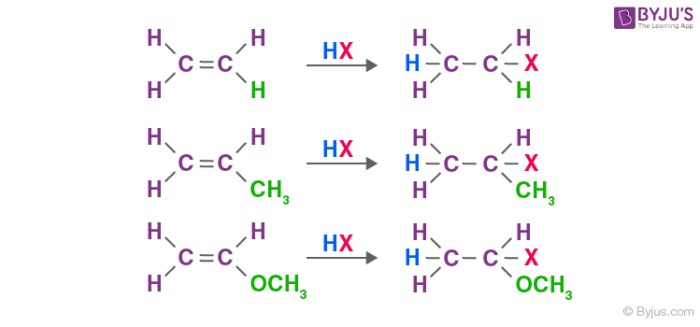
Electrophilic Addition Reactions Of Alkenes Electrophilic Substitution With Examples

Nucleophilic Addition Definition Example And Mechanism